Particle detection
Feynman photomultiplier
Feynman QED page 15.
You may wonder how it is possible to detect a single photon. The instrument capable of doing so is called a photomultiplier, and I will briefly describe how it works: When a photon strikes the metal plate A at the bottom of the diagram
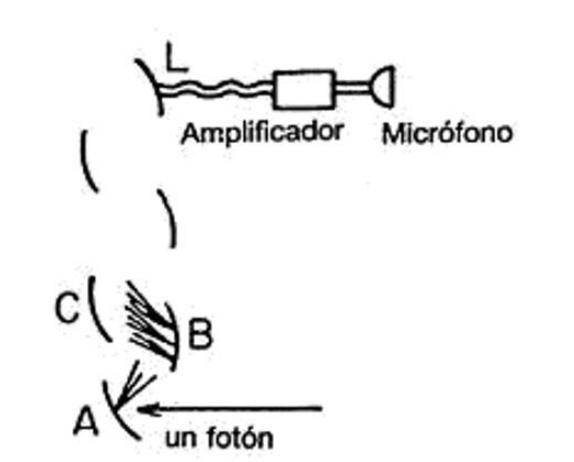
it causes an electron to be released from one of the atoms of the plate. The liberated electron is strongly attracted to plate B (which is positively charged) and strikes it with sufficient force to release three or four electrons. Each electron ejected from plate B is attracted to plate C (which is also charged) and upon colliding with it, releases more electrons in turn. This process repeats ten or twelve times, until billions of electrons, enough to generate an appreciable electric current, strike the final plate, L. This current can be amplified using a regular amplifier and sent to a speaker that produces audible clicks. Each time a photon of a specific color strikes the photomultiplier, a click of uniform volume is heard.
If we place a large number of photomultipliers around and let a very faint light shine in various directions, the light will go to one or another of the multipliers and produce a click of total intensity. It's all or nothing: if a photomultiplier fires at a given moment, no other fires simultaneously (except in the rare case of two photons leaving the light source simultaneously). There is no splitting of light into "half-particles" going to different places.
I want to emphasize that light arrives in this way—as particles. It is very important to know that light behaves like particles, especially for those of you who have gone to school, where you were probably told something about light behaving like waves. I will tell you how it really behaves—like particles.
Gas-based particle detector
I don't know if what follows it is correct (explanation by chatgpt).
Detection of a photon using a gas-based particle detector:
-
Photon Interaction with Gas: Photons, which are particles of light, have no charge. When a photon enters the particle detector and interacts with the gas inside, it doesn't directly produce a charge. However, it transfers energy to the gas atoms or molecules.
-
Ionization of Gas Atoms/Molecules: This energy transfer can ionize the gas atoms or molecules, meaning it knocks out electrons from them. These freed electrons are the start of the detection process. The photon essentially creates free electrons and positively charged ions in the gas.
-
Avalanche Effect in Gas: In the presence of a strong electric field (the accelerating potential), these free electrons gain kinetic energy. As they move rapidly through the gas, they collide with other gas atoms, ionizing them as well thanks to the kinetic energy gained, and they continue their way. This leads to more free electrons, which in turn ionize more atoms. This chain reaction is known as an avalanche effect and results in a large number of electrons and ions within a very short time.
-
Detection of the Electron Pulse: This avalanche of electrons and ions creates a current or a pulse that can be detected. The strength of this pulse is proportional to the energy of the original photon. This allows not only for the detection of the photon but also for the measurement of its energy.
-
Amplification and Measurement: The initial interaction of the photon with the gas, which resulted in just a few freed electrons, is thus amplified into a large, measurable signal. This amplified signal can then be processed and analyzed to provide information about the photon, such as its energy, and depending on the design of the detector, possibly its direction of travel.
In summary, the photon's energy is converted into an electrical signal via a cascade of interactions in the gas, which is then amplified and measured. This is how gas-based particle detectors can detect and analyze photons, which are otherwise elusive due to their lack of charge and mass.
Regarding point 3, when the electron encounter another molecule, it is true that it frees a new electro, but it is lost, itself, inside the molecule. So I cannot see how the chain reaction is achieved