Plank's law
Plank obtained a formula to explain how a body emits different lights depending of its temperature.
In this formula:
is the energy density per unit volume per unit frequency at frequency and temperature . is Planck's constant ( m kg / s). is the frequency of the electromagnetic radiation. is the speed of light in a vacuum ( m/s). is Boltzmann's constant ( m kg s K ). See Boltzmann distribution. is the absolute temperature of the black body in kelvins (K).
To understand, first, the problem solved by Plank: video of the ultraviolet catastrophe
In this video also appears the key steps in the reasoning of Plank:
Energy levels are discrete and can be represented as
To calculate average energy, replace integrals with sums.
The continuous form for average energy is (taking into account Boltzmann distribution):
This is transformed into a discrete sum for quantized energy:
Another derivation of Plank's law, here
Alternative explanation
1. Blackbody radiation
See this video. It can be studied by means of a box with a hole.

Inside the box, we have 3-dimensional standing waves at all possible frequencies (well, the corresponding wavelengths must satisfy that an integer number of half-wavelengths fit across each box dimension). In more detail, consider a rectangular box with sides of length
where
Each combination
where
This quantization in wavelengths means only discrete frequencies are allowed, but in a large box (where
On the other hand, it was observed that each frequency didn't have the same intensity. Something like this:
These graphs were empirically constructed.
2. Classical explanation using Maxwell equations
See this video
- Equipartition theorem: the equipartition theorem states that, in thermal equilibrium, the total energy of a system is distributed equally among all its degrees of freedom.
- Infinite degrees of freedom: we have an infinite number of frequencies, i.e., of degrees of freedom. Indeed, every mode of the frequency is a degree of freedom. As the frequency increases, the number of modes grows (we are in 3 dimensions!), so the energy contribution from these high-frequency modes should also increase indefinitely.
, for every mode of every wavelength. As a result, we have the ultraviolet catastrophe: much more intensity at shorter wavelengths 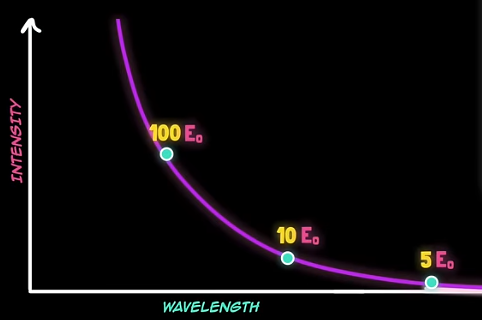
Plank's solution
For every wavelength (or frequency), he assumed that the provided energy is quantized. With a fine quantization, there is no much loss of energy, but for a coarse quantization there is a big loss. So he postulated that the quantization should be proportional to frequency (inverse to wavelength). Graphically: (this video)
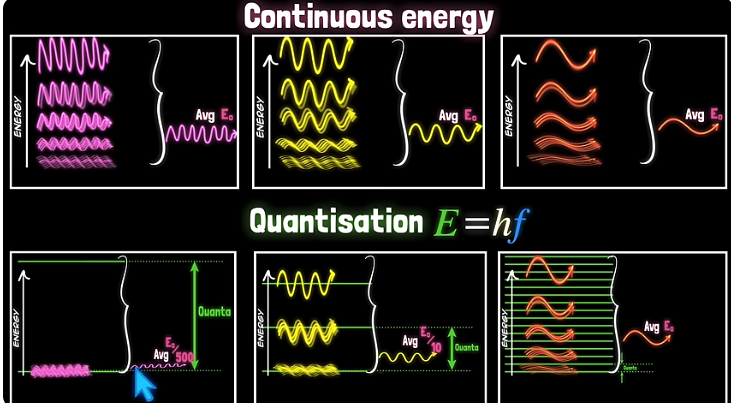
So for shorter wavelength the big loss of energy compensate to produce the observed curve, not that predicted by classical physics.
This was only a computational tool, which was explained later by Einstein together with the photoelectric effect.